- Carbon is a versatile element.
- In earth’s crust, carbon is 0.02% and found in form of minerals.
- Atmosphere has 0.03% of Carbon dioxide.
- All living structures are carbon based.
Covalent Bond in Carbon
- The atomic number of carbons is 6 and its electronic configuration is 2, 4. To attain a noble gas configuration it can
- Gain 4 electrons. But it would be difficult for nucleus to hold 4 extra electrons.
- Lose 4 electrons. But it would require a large amount of energy to remove 4 electrons.
- It is difficult thus for an atom of carbon to either gain or lose electrons.
- Carbon attains the noble gas configuration by sharing its valence electrons with other atoms. Atoms of other elements like hydrogen, oxygen, nitrogen, chlorine also show sharing of valence electrons.
- Formation of H2, O2 and N₂ is shown as below:
Join OSF Education for Offline classes.
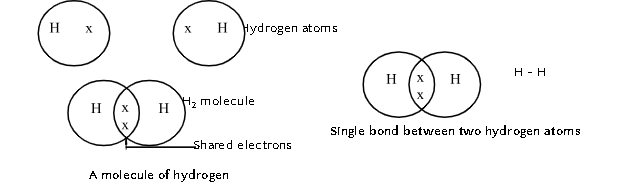
A molecule of hydrogen
Double bond
Triple bond
- It is evident that the number of shared pair of electrons can be one, two or three. Try making the structures of H2O and CH4
- Bond formed by the sharing of an electron pair between two atoms is called covalent bond.
- Covalently bonded molecules have low melting and boiling points because of comparatively weaker intermolecular forces, unlike ionic compounds.
- These molecules are generally poor conductor of electricity since no charged particles are formed.
Read other informative content here.
Versatile Nature of Carbon Atoms:
Two important properties of carbon atom enable carbon to form enormously large number of compounds.
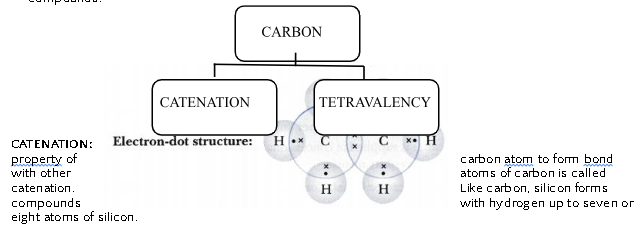
TETRAVALENCY:
Having a valency of 4, carbon atom is capable of bonding with atoms of oxygen, hydrogen, nitrogen, sulphur, chlorine and other elements.
The smaller size of carbon atom enables nucleus to hold the shared pair of electrons strongly, thus carbon compounds are very stable in general.
Saturated and Unsaturated Carbon Compounds
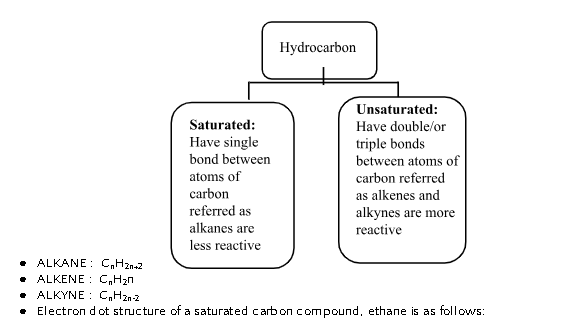
- Electron dot structure of an unsaturated carbon compound, ethane is as follows:
- Formulae and structures of saturated Compounds of Carbon and Hydrogen
On the basis of structure the hydrocarbons can be :
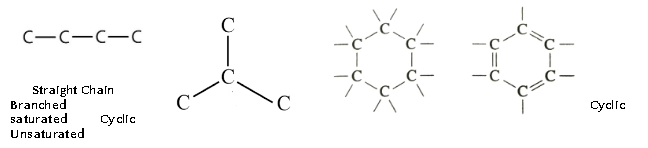
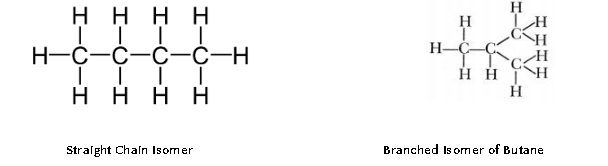
Structural isomers : these are the compounds having identical molecular formula but different structures. For example, isomers of butane.
Heteroatom and Functional Group:
In hydrocarbon chain, one or more hydrogen atoms can be replaced by other atoms in accordance with their valencies. The element that replaces hydrogen is called a heteroatom.
These heteroatoms and the group containing them impart chemical properties to the compound and hence are called functional groups.
Homologous Series:
- It is a series of compounds in which the same functional group substitutes for hydrogen in a Carbon chain.
- For instance, the ALCOHOLSS: CH3 OH, C2H5 OH, C3 H7 OH, C4 H9 OH.
- The successive member differs by -CH2-; unit and 14 units of mass.
- The chemical properties are imparted by the functional group thus all members have similar chemical properties. But the members have different physical properties.
- The physical properties vary among the members of homologous series due to difference in their molecular mass.
- Melting point and boiling point increases with increasing molecular mass.
Nomenclature of Carbon Compounds:
- Identify the number of carbon atoms in the compound.
- Functional group is indicated either by prefix or suffix .
Functional Group Suffix Prefix
| Alkene | ene | |
| Alkyne | Yne | |
| Alcohol | ol | |
| Aldehyde | al | |
| Ketone | One | |
| Carboxylic acid | Oic acid | |
| chlorine | Chloro |
- If a suffix is added, then final ‘e’ is removed from the name e.g. methanol
Chemical properties of Carbon compounds:
- COMBUSTION:
- Carbon compounds generally burn (oxidize) in air to produce carbon dioxide and water, and release heat and light energy.
- Saturated hydrocarbon burns generally with a blue flame in good supply of air and with a yellow sooty flame in limited Supply of air.
- Sooty flame is seen when unsaturated hydrocarbons are burnt.
- Burning of coal and petroleum emits oxides of sulphur and nitrogen which are responsible for acid rain.
- OXIDATION:
- Alcohols can be converted to carboxylic acids by oxidizing them using alkaline potassium permanganate or acidified potassium dichromate (they add oxygen to the reactant, thus are called oxidizing agents).
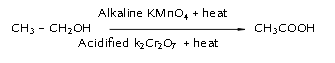
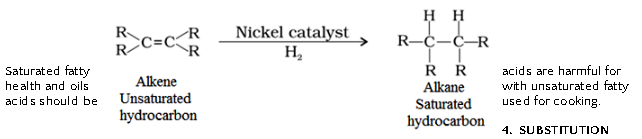
- ADDITION REACTION :
- Hydrogen is added to unsaturated hydrocarbon in presence of palladium or nickel as catalyst.
- Vegetable oils are converted into vegetable ghee using this process.
- REACTION:
- In saturated hydrocarbons, the hydrogen attached to carbon can be replaced by another atom or group of atoms in presence of sunlight.
CH4 + Cl₂→CH3CI+ HCl (sunlight required)
IMPORTANT CARBON COMPOUNDS: Ethanol and Ethanoic Acid
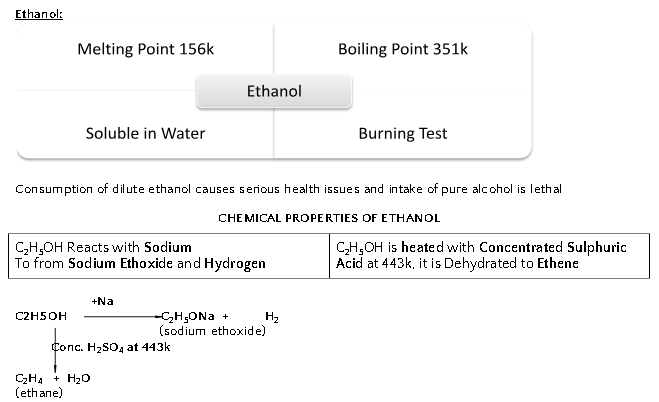
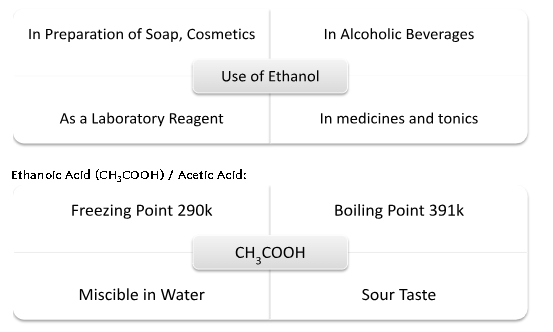
*5-8% solution of Acetic Acid in Water is called vinegar.
*Pure Acetic Acid is called glacial acetic acid.
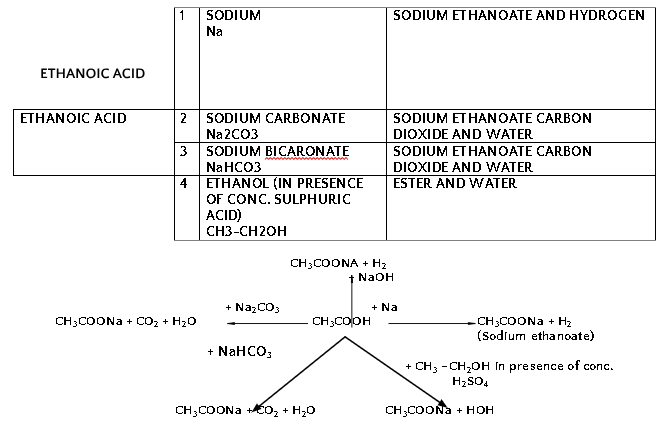
- Carboxylic acids react with alcohols in presence of few drops of concentrated sulphuric acid as catalyst and forms sweet smelling compounds called ester.
Hydrolysis:
- On heating with an acid or a base the ester forms back the original alcohol and carboxylic acid.
CH3COO CH2CH3 +NaOH→CH3COONa+ CH3-CH2OH
CH3COO CH2CH3→Heat (Dil. H2SO4) CH3COOH+CH3-CH2OH
Alkaline hydrolysis of ester is also called saponification.
Soaps and Detergents
- Soap is sodium and potassium salt of carboxylic acids with longchain.
- Soaps are effective with soft water only and ineffective with hard water.
- Detergents are ammonium or sulphonate salts of carboxylic acids with long chain. They are effective with both soft as well as hard water.
An ionic part (hydrophilic) and a long hydrocarbon chain (hydrophobic) part constitutes the soap molecule.
Structure of a Soap Molecule
Cleansing Action of Soaps:
- Most dirt is oily in nature and the hydrophobic end attaches itself with dirt, while the ionic end is surrounded with molecules of water. This result in formation of a radial structure called micelles.
- An emulsion is thus formed by soap molecule. The cloth needs to be mechanically agitated to remove the dirt particles from the cloth.
- Scum: The magnesium and calcium salts present in hard water reacts with soap molecule to form insoluble products called scum, thus obstructing the cleansing action. Use of detergents overcome this problem as the detergent molecule prevents the formation of insoluble product and thus clothes get cleaned.